
Research data encompass a wide range of information types and can differ depending on a researcher’s field of study. Research data are often the original sources or evidence collected or created during the course of a research project or study. These data form the foundation from which researchers test hypotheses, analyze trends, provide supporting evidence, draw conclusions or even refute claims.
Research data may take many forms, including but not limited to: laboratory notebooks, spreadsheets, genomic sequences, environmental measurements, audio or visual recordings, photographs, imaging, spatial data, and sensor data. If a funding agency requires a formal Data Management Plan (DMP), it will often provide guidance on what it considers to be data.
The Federal government defines ‘data’ in OMB Circular A-110 (now 2 CFR, Ch. II, §215.36(d)(2)(i) as: the recorded factual material commonly accepted in the scientific community as necessary to validate research findings. In turn, many federal agencies, such as the NSF and NIH, have adopted this definition and include it in their data management and sharing policies. For more information on these policies, visit the Federal Funding Guideline tab in this guide.
Research Data Management (RDM) is the process of planning, organizing, and documenting how research data will be collected, structured, analyzed, stored, and shared. As a project evolves, strategies for managing data may shift to meet the needs of different research phases. Documenting these plans, in a data management plan or similar format, is essential. Such documentation supports continuity when team members join or leave, and ensures data remains understandable and usable long after the project ends.
A wide range of resources are available to help researchers understand data management and the key considerations involved in planning for data throughout the research process. Many of these resources are freely accessible to the public and linked throughout this guide. For UNLV researchers, please visit the Developing Your Data Management Plan webpage as well as the Writing Data Management Plans and Resources for UNLV Researchers tabs in this guide.
Research data management (RDM) involves the thoughtful planning and careful handling of data across all stages of the research data lifecycle, starting from conception and proposal writing to data collection, publication, and long-term preservation. Effective RDM practices vary by discipline and research type, but are essential for ensuring data quality, integrity, reproducibility, and accessibility.
|
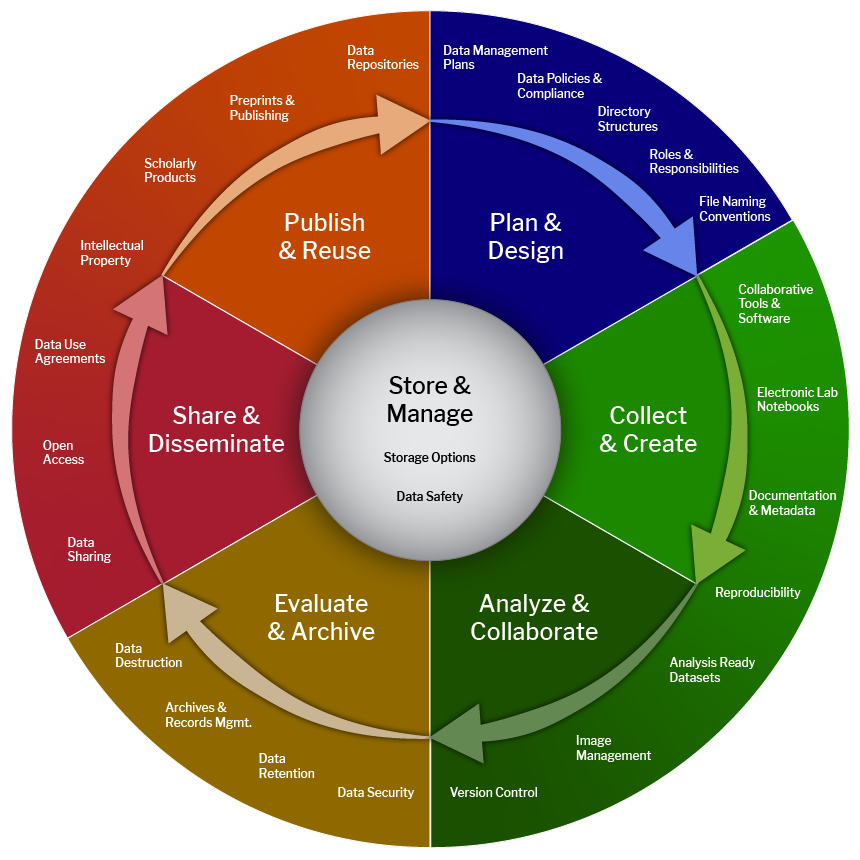
Plan & Design: Plan processes from onboarding to project closure and data resources Collect & Create: Organization and integration of data sets and collection processes Analyze & Collaborate: Processing and analyzing data should be collaborative and documented Store & Manage: Each stage of the Biomedical Data Lifecycle revolves around the management of data storage Evaluate & Archive: Identify essential research records and evaluate for retention Share & Disseminate: Establishing and supporting the reach and impact of your data Publish & Reuse: Ensuring the broad utility of your research data efforts for other researchers |
|---|
Data Lifecycle from the LMA Research Data Management Working Group
